Abstract
Background: Real-world evidence highlighting the tx landscape and outcomes among pts with CLL/SLL who have received ≥3 lines (3L+) of systemic therapy that include a prior BTKi and/or BCL2i are limited. The objective of this study was to describe pt characteristics, tx patterns, and overall survival (OS) among these pts.
Methods: This was a retrospective study using de-identified, electronic health records from the Flatiron Health database from 01/01/2011 to 02/28/2022. The study population included US pts aged ≥ 18 years with CLL/SLL who received ≥ 2 prior lines of therapy (LOT) including a BTKi (cohort 1) or both a BTKi and a BCL2i (cohort 2) and who initiated a subsequent LOT. For cohorts 1 and 2, respectively, the index date was defined as the start of 3L+ therapy after discontinuation of a BTKi and discontinuation of a BTKi and a BCL2i. Pts with any other primary cancers (except nonmelanoma skin cancer) within 12 months before the index date or during the follow-up period were excluded from the analytic cohorts. Descriptive analyses were conducted to examine pt characteristics and tx patterns. Time to discontinuation (TTD), time to next tx (TTNT), and OS for the index LOT were assessed using Kaplan-Meier (KM) method. TTD was defined as the time between index therapy start date and end date. TTNT was defined as the time between start of index therapy and start of subsequent LOT.
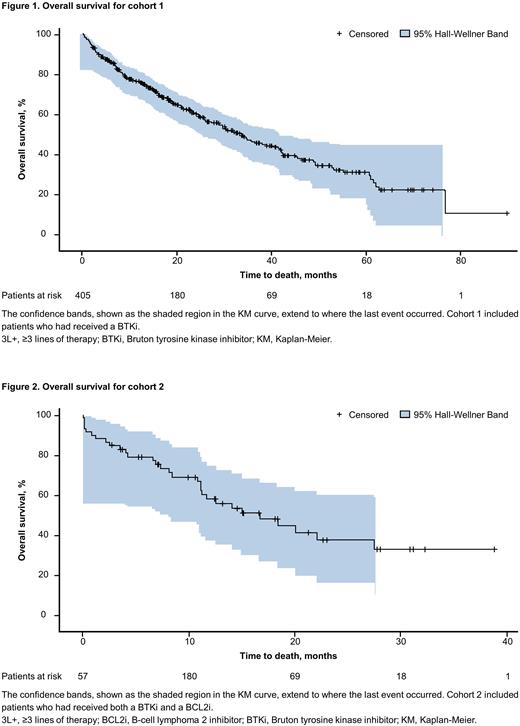
Results: The study sample included 405 pts in cohort 1 and 57 pts in cohort 2, with a median follow-up of 18 and 11 months, respectively. The majority of pts (percent in cohort 1 and cohort 2) were aged ≥ 65 years (80.2% and 78.9%), male (60.2% and 50.9%), White (76% and 78.9%), and treated in the community setting (93.6% and 94.7%). Approximately 70% of pts in cohort 1 began their index LOT in 2018 or later, whereas 79% of pts in cohort 2 began their index LOT in 2019 or later. Pts in both cohorts were largely well functioning, with ECOG PS score ≤ 1 in 83.7% of cohort 1 and 68.0% of cohort 2. Disease transformation was reported among 8.9% of pts in cohort 1 and 14.0% of pts in cohort 2. Pts in cohort 1 received a median of 2 prior LOTs, whereas those in cohort 2 received a median of 3 prior LOTs. The most common txs observed in cohort 1 in the index LOT were BTKi monotherapy (20.0%), chemotherapy + anti-CD20 mAb (19.0%), BCL2i monotherapy (13.3%), and BCL2i + anti-CD20 mAb (13.1%). The most common txs observed in cohort 2 in the index LOT were BCL2i + anti-CD20 mAb (19.3%), phosphatidylinositol-3-kinase inhibitor monotherapy (17.5%), chemotherapy + anti-CD20 mAb (14.0%), and BCL2i monotherapy (12.3%). Median (95% CI) KM estimate of TTD for index therapy was 13.2 (11.1‒17.5) months and 7.1 (5.0‒11.8) months for cohorts 1 and 2, respectively. For pts who received a subsequent LOT after index therapy, the median (95% CI) KM estimate of TTNT was 11.3 (9.1‒12.6) months for cohort 1 and 6.6 (3.6‒10.1) months for cohort 2. The median (95% CI) OS was 33.1 (28.3‒40.0) months (Figure 1) and 16.6 (11.0‒27.5) months (Figure 2), and the 12-month OS estimate was 76.5% and 57.5%, for cohorts 1 and 2, respectively.
Conclusions: The study findings highlight a significant unmet need in pts with 3L+ CLL/SLL. Real-world tx patterns observed in this study are reflective of a heterogenous tx landscape without the presence of a standard of care. The results indicate that pts receive similar txs as used in earlier LOTs. This rechallenging results in suboptimal disease control as indicated by short TTNT, TTD, and estimated OS. Study findings suggest that disease prognosis worsens among pts who were heavily pretreated (i.e., cohort 2, which received prior BTKi and BCL2i), despite the relatively small sample size. Although response and disease progression data were not captured in the Flatiron database, this study sufficiently demonstrates the need for newer and more effective options in the CLL/SLL tx landscape, which can delay disease progression while ensuring better disease control. Overall, these findings are generalizable to pts with CLL/SLL treated in the community setting in the US.
Disclosures
Awan:AbbVie: Consultancy; Verastem: Consultancy; Cardinal Health: Consultancy; Merck: Consultancy; BMS: Consultancy; Incyte: Consultancy; MEI Pharma: Consultancy; ADCT Therapeutics: Consultancy; Pharmacyclics: Consultancy, Research Funding; Gilead Sciences: Consultancy; Kite Pharma: Consultancy; Karyopharm: Consultancy; Cellecter Bisosciences: Consultancy; Dava Oncology: Consultancy; Johnson and Johnson: Consultancy; BeiGene: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Epizyme: Consultancy; AstraZeneca: Consultancy; Genentech: Consultancy; Caribou Biosciences: Consultancy. Deshpande:BMS: Current Employment, Current equity holder in publicly-traded company. Le:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Garrison:BMS: Current Employment, Current equity holder in publicly-traded company. Tiwana:BMS: Current Employment, Current equity holder in publicly-traded company. Priyadarshini:BMS: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.